Electroreception, is the biological ability to perceive electrical
impulses. It is an ancient sense that has evolved independently
across the animal kingdom in multiple groups including agnathan
(lampreys), cartilaginous (chimaeras, sharks, skates/rays) and bony
fishes (lungfish, coelacanth, polypterids, chondrosteans and
teleosts), some amphibians and mammals.
The multiple and independent evolution of electroreception
emphasises the importance of this sense in a variety of aquatic
environments. The electrosensory system of sharks is comprised of a
series of electroreceptors, known as the ampullae of Lorenzini,
distributed over almost the entire surface of the head.
It is thought that the major role of the electroreceptors is
in the detection of prey, with other functions, including the
detection of predators, facilitating social behaviours and
orientation to the earth’s magnetic field for navigation.
The Discovery of Electroreception
 It is believed that the "electric" fish evolved from a pre-electric
fish without electric organs but sensitive to electric fields
(Bennett, 1970).
Furthermore, it is suggested that at that primitive stage, the
electrosensitivity might have been used to detect the muscular
potentials of prey, predators, and members of the same species
(Kalmijn, 1971).
It is believed that the "electric" fish evolved from a pre-electric
fish without electric organs but sensitive to electric fields
(Bennett, 1970).
Furthermore, it is suggested that at that primitive stage, the
electrosensitivity might have been used to detect the muscular
potentials of prey, predators, and members of the same species
(Kalmijn, 1971).
In 1917, Parker and Van Heusen published a paper on the behavioural
responses of the catfish, Ameiurus nebulosus, to metallic and
non-metallic rods (Parker and Van Heusen, 1917).
They reported sensitivity of blindfolded catfish to metallic
rods but not to glass rods until the glass rod came in contact with
its skin, which then invoked a response.
Parker and Van Heusen did not realise it at the time, but
they were studying the electrosensitivity of fish that have distinct
electroreceptors (Kalmijn, 1971).
The first evidence of electrosensitivity in Elasmobranchs dates back
to 1935 when Dijkgraaf, working on Scyliorhinus canicula,
noticed the animal's sensitivity to a rusty steel wire (Kalmijn and
Dijkgraaf, 1962). The experimenters approached the head of a
blindfolded shark with such a wire, just as Parker and Van Heusen
did with the catfish (Parker and Van Heusen, 1917).
They observed that the animal escaped when the wire was
closer than several centimeters from its head.
They repeated the experiment with a glass rod, which the
animal did not react to it.
Dijkgraaf assumed that the shark was stimulated by the
galvanic currents produced at the surface of the metal wire, but had
no way of proving his assumption (Kalmijn and Dijkgraaf, 1962).
 Dijkgraaf's hypothesis largely remained a speculation until Lissmann
(1958) formally suggested, based on behavioural evidence, that a
group of receptors and central processes, called the ampullae of
Lorenzini, aid in the detection and analysis of electric fields in
the marine environment of fish (Lissmann, 1958).
Lissmann suggested that ‘weakly electric fish’ evolved from
pre-electric fish with no electric organs but which were already
sensitive to electric fields (Lissmann, 1958).
He proposed that muscle potentials, such as that from a
regular heartbeat of prey, a predator, a member of the same species
or from the individual itself, may have been detected by this early
form of electrosensitivty (Lissmann, 1958).
His proposal seems quite conceivable as there are fish living
today (catfish and sharks) that are very sensitive to electric
fields but lack electric organs (Kalmijn, 1971).
The hypothesis that muscle potentials are used to detect the
location of other animals was also supported by the findings of
Kalmijn (1966).
Dijkgraaf's hypothesis largely remained a speculation until Lissmann
(1958) formally suggested, based on behavioural evidence, that a
group of receptors and central processes, called the ampullae of
Lorenzini, aid in the detection and analysis of electric fields in
the marine environment of fish (Lissmann, 1958).
Lissmann suggested that ‘weakly electric fish’ evolved from
pre-electric fish with no electric organs but which were already
sensitive to electric fields (Lissmann, 1958).
He proposed that muscle potentials, such as that from a
regular heartbeat of prey, a predator, a member of the same species
or from the individual itself, may have been detected by this early
form of electrosensitivty (Lissmann, 1958).
His proposal seems quite conceivable as there are fish living
today (catfish and sharks) that are very sensitive to electric
fields but lack electric organs (Kalmijn, 1971).
The hypothesis that muscle potentials are used to detect the
location of other animals was also supported by the findings of
Kalmijn (1966).
By 1957,  Through the culmination of various studies, Murray finally
proposed an electrosensory function for the ampullae of Lorenzini in
1960, when he found that the ampullae in rays, such as Raja
clavata, were sensitive to slight changes in the electrical
potential field surrounding them (Murray, 1960).
A study by Dijkraaf and Kalmijn (1963) then subsequently
demonstrated this and behavioural studies by Kalmijn (1971)
supported them and demonstrated that it was the ampullae of
Lorenzini, and only these, that were responsible for the sensitivity
of Elasmobranchs to electric fields, designating them as the
electroreceptors of Elasmobranchs.
More recent studies have also suggested that the ampullary
system may be used for orientation and navigation (Kalmijn, 1984,
1988; Paulin 1995; Fishelson and Baranes, 1998; Montgomery and
Walker, 2001) as well as for the detection of individuals in social
behaviours (Sisneros et al., 1998).
Although to date the primary importance of the
electroreceptors is believed to be their use in the detection and of
prey (Kalmijn, 1971).
Through the culmination of various studies, Murray finally
proposed an electrosensory function for the ampullae of Lorenzini in
1960, when he found that the ampullae in rays, such as Raja
clavata, were sensitive to slight changes in the electrical
potential field surrounding them (Murray, 1960).
A study by Dijkraaf and Kalmijn (1963) then subsequently
demonstrated this and behavioural studies by Kalmijn (1971)
supported them and demonstrated that it was the ampullae of
Lorenzini, and only these, that were responsible for the sensitivity
of Elasmobranchs to electric fields, designating them as the
electroreceptors of Elasmobranchs.
More recent studies have also suggested that the ampullary
system may be used for orientation and navigation (Kalmijn, 1984,
1988; Paulin 1995; Fishelson and Baranes, 1998; Montgomery and
Walker, 2001) as well as for the detection of individuals in social
behaviours (Sisneros et al., 1998).
Although to date the primary importance of the
electroreceptors is believed to be their use in the detection and of
prey (Kalmijn, 1971).
The Physical Stimuli for
Electroreception
In the oceans, electric fields are induced by both biological and
geological causes. In
the latter case electric fields are induced by water flowing or fish
swimming through the earth's magnetic field by geomagnetic
variations (the fluctuating strength of the earth's magnetic field)
and by geophysical events, such as the tectonic processes that cause
strain variations in the earth's crust which lead to changes in the
magnetization of rocks and local electric fields (Kalmijn, 1988).
It is thought that Elasmobranchs are able to utilise these
electric fields for navigation and identification of their
environment (Kalmijn, 1984).
 Electric fields in the oceans can also be produced by marine animals
(Kalmijn, 1974). The internal electrochemical environments of marine
animals differ from the external, which creates a difference in the
voltage gradient across the water skin boundary (Kalmijn, 1974).
The potential difference produces current loops which yield a
bioelectric field in the surrounding waters.
An organism’s behaviour will also produce additional electric
fields in the surrounding water (Kalmijn, 1974).
For example, when a fish swims, muscles contract, muscle
contraction takes place when chemically-dependent channels,
impermeable to sodium and potassium, open.
The movement of such ions across the membrane produces an
electric field that travels away from the individual in the
conducting medium (salt water) (Kalmijn, 1974).
Electric fields in the oceans can also be produced by marine animals
(Kalmijn, 1974). The internal electrochemical environments of marine
animals differ from the external, which creates a difference in the
voltage gradient across the water skin boundary (Kalmijn, 1974).
The potential difference produces current loops which yield a
bioelectric field in the surrounding waters.
An organism’s behaviour will also produce additional electric
fields in the surrounding water (Kalmijn, 1974).
For example, when a fish swims, muscles contract, muscle
contraction takes place when chemically-dependent channels,
impermeable to sodium and potassium, open.
The movement of such ions across the membrane produces an
electric field that travels away from the individual in the
conducting medium (salt water) (Kalmijn, 1974).
The number of muscle contractions affects the magnitude of the
electric fields. If
more muscles contract, the magnitude of the field is greater and
vice versa (Kalmijn, 1974).
Furthermore, the intensity of the electric fields changes in
the case of a wounded animal.
For example, Kalmijn (1974) measured that crustaceans can
generate a voltage of 50.0 mV measured with a sensing electrode 1 mm
away from the surface of the animal.
The same crustacean, if wounded, was shown to generate a much
higher voltage of 1250.0 mV (Kalmijn, 1974).
It was way back in 1947 when Burr originally established the
presence of these bioelectric fields in the vicinity of marine
animals, but relatively little work was done on the bioelectrics of
marine animals until Kalmijns work in the 70’s (Kalmijn, 1974).
The voltage changes shown by Kalmijn (1974) have been shown
to be easily detected by members of Elasmobranchs.
The Ampullae of Lorenzini
 The ampullae of Lorenzini are jelly-filled canals found on the head
of Elasmobranchs which form a system of sense organs, each of which
receives stimuli from the outside environment through the dermis and
epidermis (Raschi et al. 1997).
The canals range anywhere from 1 to 25 cm in length for
Elasmobranchs, and are approximately 0.1 cm in diameter (Fig. 1)
(Brown et al., 2005).
Each canal ends in groups of small bulges lined by the
sensory epithelium. A
small bundle of afferent nerve fibres stimulate each ampullae; there
are no efferent fibres (
The ampullae of Lorenzini are jelly-filled canals found on the head
of Elasmobranchs which form a system of sense organs, each of which
receives stimuli from the outside environment through the dermis and
epidermis (Raschi et al. 1997).
The canals range anywhere from 1 to 25 cm in length for
Elasmobranchs, and are approximately 0.1 cm in diameter (Fig. 1)
(Brown et al., 2005).
Each canal ends in groups of small bulges lined by the
sensory epithelium. A
small bundle of afferent nerve fibres stimulate each ampullae; there
are no efferent fibres (
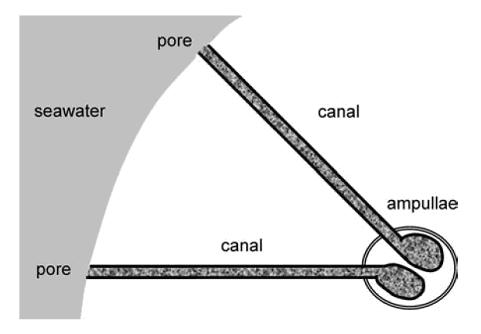
Figure 1.
Sketch of two electrosensitive organs and their associated canals in
a marine Elasmobranch, where gray regions denote the seawater
environment and speckled regions denote the hydrogel. The individual
ampullae here are simplified: each contains multiple chambers, or
alveoli, and neither the sensing cells of the ampullae nor their
associated afferent nerve fibres are shown (Brown et al.,
2005).
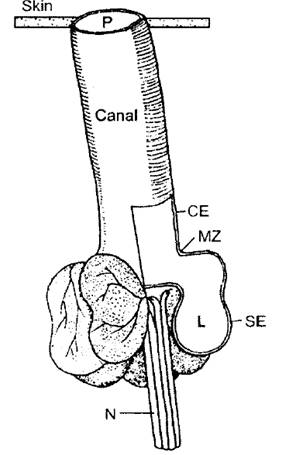
Figure 2.
Diagram of the ampullae of Lorenzini, formed by several alveoli that
share a continuous lumen (L) and a subdermal canal that has a single
pore on the skin. The
sensory epithelium (SE) forms the highly resistive ampullae wall
that connects with the canal epithelium (CE) at the marginal zone
(MZ). The sensory
epithelium is innervated by primary afferent neurons (N) that
conduct electrosensory information to the brain (Tricas, 2001).
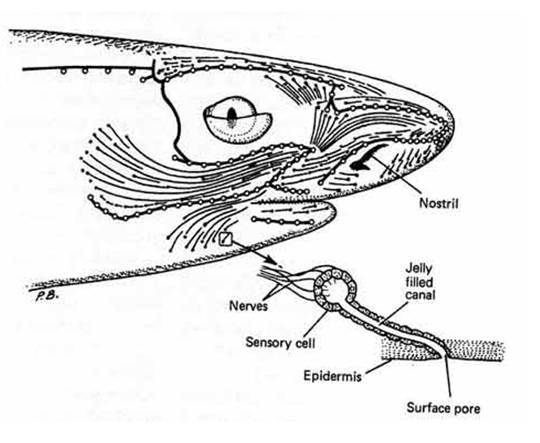
Figure 3.
Diagram to illustrate the surface area of the head of a Squaloid
shark which is covered with the electrosensory system with a
magnified illustration of an individual ampullae, demonstrating how
each cell is innervated by primary afferent neurons (Compagno et
al., 2004).
Arrangement of the Ampullary
of Lorenzini
 The morphological arrangement of the ampullary canals permits
detection of both small local electric fields produced by biological
organisms and large uniform electric fields of inanimate or animate
origins (Kalmijn 1974).
When a small localized dipole stimulus (such as that of a small
prey) is presented at a pore that is far away from its ampullae, the
potential is conducted to receptor cells within the ampullae chamber
(Fig. 1-3). However,
when the animal’s body is within a uniform field (or at the fringe
of a large dipole field) the body can admit a portion of the field
that can influence the internal reference potential.
When the weak uniform electric field is parallel to the
canal, the stimulus voltage at the apical surface of receptor cells
is determined by the linear separation between the ampullae and its
canal pore. Thus, long
canals sample across a greater distance within the field and provide
a larger potential difference for receptor cells than do ampullae
with short canals (Tricas, 2001).
In addition, the strongest potential difference occurs when
the canal is oriented parallel to the field and decreases as a
cosine function as it deviates away from the direction of the field
(Tricas, 2001).
Therefore, when an omnidirectional ampullary array is within a
uniform field, the canals simultaneously sample the external
potentials at different points on the body.
Theoretically this can provide immediate information about
the field’s intensity, spatial configuration and possibly the
direction of the source (Tricas, 2001).
The morphological arrangement of the ampullary canals permits
detection of both small local electric fields produced by biological
organisms and large uniform electric fields of inanimate or animate
origins (Kalmijn 1974).
When a small localized dipole stimulus (such as that of a small
prey) is presented at a pore that is far away from its ampullae, the
potential is conducted to receptor cells within the ampullae chamber
(Fig. 1-3). However,
when the animal’s body is within a uniform field (or at the fringe
of a large dipole field) the body can admit a portion of the field
that can influence the internal reference potential.
When the weak uniform electric field is parallel to the
canal, the stimulus voltage at the apical surface of receptor cells
is determined by the linear separation between the ampullae and its
canal pore. Thus, long
canals sample across a greater distance within the field and provide
a larger potential difference for receptor cells than do ampullae
with short canals (Tricas, 2001).
In addition, the strongest potential difference occurs when
the canal is oriented parallel to the field and decreases as a
cosine function as it deviates away from the direction of the field
(Tricas, 2001).
Therefore, when an omnidirectional ampullary array is within a
uniform field, the canals simultaneously sample the external
potentials at different points on the body.
Theoretically this can provide immediate information about
the field’s intensity, spatial configuration and possibly the
direction of the source (Tricas, 2001).
Distribution of the Ampullary
of Lorenzini
The spatial organisation of the ampullae of Lorenzini is largely
determined by body morphology, for example, the dorso-ventrally
flattened body of the Batoids restricts the canals in the horizontal
plane (Tricas, 2001).
Recent studies now believe that it is also related to feeding
preferences and migratory habits (Raschi, 1978; 1986; Kajiura, 2001;
Kajiura and Holland, 2002; Atkinson and Bottaro, 2006).
Species showing reduced development of the ampullary system,
represented by low pore counts, appear to have well-developed eyes
suggesting a greater dependence on vision.
More migratory species appear to have a more even
distribution of pores over both the dorsal and ventral surfaces and
those feeding predominantly on benthic prey possessed larger numbers
of pores on their ventral surface (Raschi et al., 2001).
A study by Raschi (1986) on skates showed that specimens predating
upon more active prey possessed more of an even pore distribution
over the dorsal and ventral surfaces, whereas those feeding on more
sedentary prey possessed a greater abundance of pores on the ventral
surface. The study
recorded that species feeding on more sedentary prey would generally
position themselves directly over the prey before they strike to
consume it. Greater
number of pores on the ventral surface, particularly around the
buccal region, is therefore believed to guide the mouth in for the
final feeding strike by providing a greater resolution for locating,
manipulating and ingesting prey.
Species feeding on more active prey initiate their feeding
strike at a greater distance, relying more on vision and possessing
a more even distribution of pores to provide readings of stimuli
from all regions of the head (Raschi, 1986).
Pore
Mapping
 Kajiura (2001) looked at the distribution of electrosensory pores on
two Sphyrnids, the scalloped hammerhead (Sphryna lewini), the
bonnethead (Sphryna tiburo), and a representative
Carcharhinid, the sandbar shark (Carcharhinus plumbeus).
Head morphology, pore number and pore density were quantified
to test the assumption and predictions of enhanced electrosensory
pore abundance in hammerhead sharks.
The assumption of Kajiura (2001) was that the hammerheads
would have their electroreceptors extended over a greater lateral
distance than the Carcharhinids.
Obviously Kajiura’s (2001) assumption was true due to the
distinct head morphology of the Sphyrnids.
Both of the Sphyrnid species have greater head width than the
sandbar shark. The
electrosensory pores were shown to be distributed across the entire
surface of the head for all species in the investigation (Kajiura,
2001). Thus, the
electroreceptors of the Sphyrnids are distributed over a greater
lateral distance. One
of the factors which drove evolution of the cephalofoil (The unique
cranial morphology of the ‘hammerhead’ shark) might have been
selection for a head in which the electroreceptors were spaced
further apart to increase the amount of lateral area sampled by the
head (Kajiura, 2001; Kajiura et al., 2005).
A larger head would increase foraging efficiency by allowing
the shark to search a larger area of the benthos.
A 1m (Precaudal length) sandbar shark has a head width
equivalent to a hammerhead of only 37 cm (Precaudal length). Thus, a
small hammerhead would be able to search the same lateral area as a
sandbar shark that is 2.7 times as long (Kajiura, 2001).
Kajiura (2001) looked at the distribution of electrosensory pores on
two Sphyrnids, the scalloped hammerhead (Sphryna lewini), the
bonnethead (Sphryna tiburo), and a representative
Carcharhinid, the sandbar shark (Carcharhinus plumbeus).
Head morphology, pore number and pore density were quantified
to test the assumption and predictions of enhanced electrosensory
pore abundance in hammerhead sharks.
The assumption of Kajiura (2001) was that the hammerheads
would have their electroreceptors extended over a greater lateral
distance than the Carcharhinids.
Obviously Kajiura’s (2001) assumption was true due to the
distinct head morphology of the Sphyrnids.
Both of the Sphyrnid species have greater head width than the
sandbar shark. The
electrosensory pores were shown to be distributed across the entire
surface of the head for all species in the investigation (Kajiura,
2001). Thus, the
electroreceptors of the Sphyrnids are distributed over a greater
lateral distance. One
of the factors which drove evolution of the cephalofoil (The unique
cranial morphology of the ‘hammerhead’ shark) might have been
selection for a head in which the electroreceptors were spaced
further apart to increase the amount of lateral area sampled by the
head (Kajiura, 2001; Kajiura et al., 2005).
A larger head would increase foraging efficiency by allowing
the shark to search a larger area of the benthos.
A 1m (Precaudal length) sandbar shark has a head width
equivalent to a hammerhead of only 37 cm (Precaudal length). Thus, a
small hammerhead would be able to search the same lateral area as a
sandbar shark that is 2.7 times as long (Kajiura, 2001).
Methods Used in Pore
Mapping
Fishelson et al. (1998) and Whitehead et al. (1999)
determined the distribution of ampullae by total body staining with
methylene blue, found to be an effective in vitro stain for the
ampullae of Lorenzini.
Rinsing off excess stain on the skin revealed the distribution of
the various pores, specifically of the ampullae.
As the stain was also taken up by the mucus inside the tubuli
of these organs but not by the canals of the lateral line system,
this facilitated mapping of pore distribution on the head.
It also enabled the number of ampullary alveoli to be counted
(after the removal of the dermis) on various sites of the head.
Kajiura (2001) compared the electrostatic pore distribution patterns
of carcharhinid and sphyrnid sharks, by taking a representative head
from each species and carefully dissecting the dermis from the head.
The intact dermis was cut along the frontal plane to divide the
dermis into dorsal and ventral halves which were cleaned of
subdermal tissue. Each
dermal sample was sandwiched between panes of glass, backlit by
natural sunlight and photographed with colour slide film.
Pores appeared as bright points of light against a dark
background of skin. The
photographic slides (35 mm) of each skin sample were projected onto
paper, the head outline traced and each pore mapped.
The final product was a direct one to one correspondence map
of pores on a head (Fig. 4, 5).
Atkinson (2003) took a much simpler approach to pore mapping, which
involved dividing the head of two shark species (Etmopterus
spinax and Galeus melastomus) in to specific regions,
including ventral, dorsal, lateral and buccal.
The number of pores were then numerated and compared by
region. Although this
seemed to be a good approach for comparing distribution of pores
between species, Atkinson’s description of the position of the
regions was less than precise and as a result difficult to
replicate.
One of the problems associated with the study of electrosensory
pores distribution has been that there is no definitive methodology
used in the mapping process.
Thus, it is often the case that the results from different
investigations are not comparable.
In a study by Fishelson and Baranes (1998) the pores
identified for the Oman Shark, Iago omanensis, were grouped
by where the largest assemblages were seen, the groups were named by
the region of the head, in which they are found.
The dorsal side of the head featured pairs of mediorostral,
laterorostral, and preorbital groups and one frontal group, situated
at the base of the rostrum in front of the eyes.
The ventral side possessed only two, small mandibular groups.
Fishelson and Baranes (1998) method seems perfectly accurate
and achievable but when you compare it, for example, to Atkinson’s
(2003) work it is difficult to appreciate where there may be
similarities between species due to the different counting methods
adopted by each researcher.
Patterns in the Distribution of
Electrosensory Pores
Kajiura’s (2001) investigation showed the number and distribution of
pores on the dorsal and ventral surfaces of three shark species.
The species in question were the scalloped hammerhead (S.
lewini), bonnethead (S. tiburo) and sandbar shark (C.
plumbeus). The
scalloped hammerhead had the greatest number of pores on the ventral
surface of the head and yielded a mean dorsal to ventral pore ratio
of 0.71. The bonnethead
shark had a mean ratio of 0.84.
Although both Sphyrnid species had a greater number of pores
on the ventral surface of the head, C. plumbeus had a
distribution of pores close to equal on dorsal and ventral surfaces
with a ratio of 1.05.
Despite the differences in the number of pores on dorsal and ventral
surfaces between the Sphyrnid and the Carcharhinids sharks.
Kajiura (2001) showed that the general pattern of pore field
distribution on the head is conserved across the three examined
species (Figures 4).
The pore distribution pattern on the dorsal surface of the head was
divided into four pore fields (Figure 2) and the pore distribution
pattern on the ventral surface of the head was divided into eight
pore fields (Figure 3).
Kajiura (2001) showed that the percentage of pores in each of the
pore fields was mostly comparable between each of the three species.
The notable exceptions included a greater number of pores in
section b for the sandbar shark and a greater number of pores in
section j for the scalloped hammerhead.
In both cases the bonnethead shark displayed an intermediate
value.
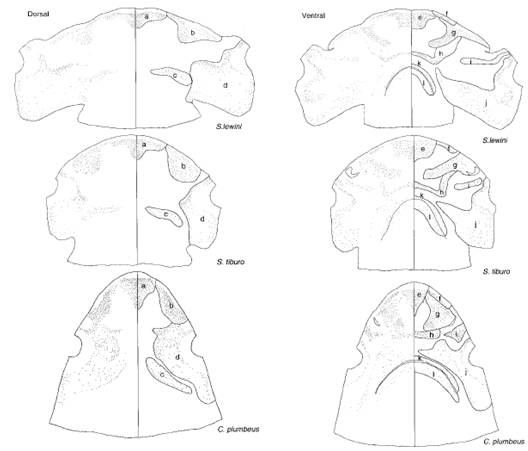
Figure 4
Distribution pattern of electroreceptor pores on the dorsal and
ventral surface of the head of scalloped hammerhead, bonnethead and
sandbar shark. Pores are illustrated on the entire dorsal and
ventral surface and the right side of each head is subdivided into
pore fields which correspond across species. (Kajiura, 2001)
Physical Characteristics
of the Ampullae of Lorenzini
In one experiment
Role of
Electroreception in Feeding behaviour
 In 1971 Kalmijn looked at the feeding responses of the shark,
Scyliorhinus canicula, and the ray, Raja clavata, toward
the flatfish, Pleuronectes platessa.
Kalmijn’s (1971) experiments demonstrated that Elasmobranchs
make significant use of their sensitivity towards electric fields.
First, the flatfish was introduced into a pool where the
sharks and rays were maintained, and the flatfish was given enough
time to bury itself in the sand. When the sharks and rays swam
within 10-15 cm of the flatfish, they would attack the spot where
the fish was buried.
Subsequently, the flatfish was retrieved and consumed by the sharks
and rays. Kalmijn
(1974) then placed a flatfish in an agar chamber to conceal it both
mechanically and chemically from the sharks and rays without
affecting its electric field.
Kalmijn (1974) noted no change in the attack pattern of the
sharks and rays. To
prove that the 1 cm agar layer was thick enough to block the
chemical scent of the flatfish, frozen pieces of fish were used
instead. Following this
change, neither the sharks nor rays attacked the chamber.
Next, the live flatfish was returned to the agar chamber and
a thin electrically-insulating plastic film was placed above the
chamber to block the electric field of the flatfish.
Once again the sharks and rays made no attempt to attack the
flatfish. Finally, to
provide direct evidence for the sharks and rays ability to detect
electric fields, two electrodes were buried under the sand, and a
current was passed between them.
The shark and ray exhibited the same attack pattern as when a
live flatfish was buried under the sand (Kalmijn, 1974).
In 1971 Kalmijn looked at the feeding responses of the shark,
Scyliorhinus canicula, and the ray, Raja clavata, toward
the flatfish, Pleuronectes platessa.
Kalmijn’s (1971) experiments demonstrated that Elasmobranchs
make significant use of their sensitivity towards electric fields.
First, the flatfish was introduced into a pool where the
sharks and rays were maintained, and the flatfish was given enough
time to bury itself in the sand. When the sharks and rays swam
within 10-15 cm of the flatfish, they would attack the spot where
the fish was buried.
Subsequently, the flatfish was retrieved and consumed by the sharks
and rays. Kalmijn
(1974) then placed a flatfish in an agar chamber to conceal it both
mechanically and chemically from the sharks and rays without
affecting its electric field.
Kalmijn (1974) noted no change in the attack pattern of the
sharks and rays. To
prove that the 1 cm agar layer was thick enough to block the
chemical scent of the flatfish, frozen pieces of fish were used
instead. Following this
change, neither the sharks nor rays attacked the chamber.
Next, the live flatfish was returned to the agar chamber and
a thin electrically-insulating plastic film was placed above the
chamber to block the electric field of the flatfish.
Once again the sharks and rays made no attempt to attack the
flatfish. Finally, to
provide direct evidence for the sharks and rays ability to detect
electric fields, two electrodes were buried under the sand, and a
current was passed between them.
The shark and ray exhibited the same attack pattern as when a
live flatfish was buried under the sand (Kalmijn, 1974).
The experiments conducted by Kalmijn (1974) suggest that detection
of electric fields directly influences the feeding response of
Elasmobranchs. The
behavioural evidence combined with the ability of Elasmobranchs to
detect electric fields in their natural environment leads to the
conclusion that electroreception is a biologically significant
modality to these organisms (Kalmijn, 1974).
The great advantage Elasmobranchs have over other organisms
has made them into one of the most threatening and successful
predators on earth.
